Why an Iowa City hotspot could help UI researchers more effectively study a COVID-19 vaccine
The University of Iowa has nearly hit its goal of enrolling 250 volunteers in a trial for a COVID-19 vaccine from Pfizer and BioNtech. When research would be wrapped and a vaccine administered, however, remains a question mark.
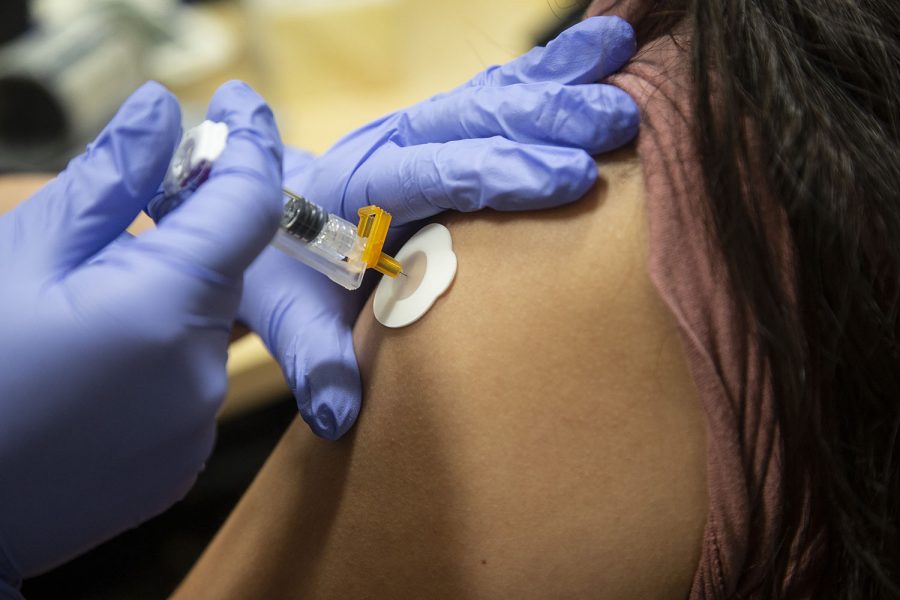
Photo Illustration by Katie Goodale
September 15, 2020
The University of Iowa — home to a top medical college and now a national COVID-19 hotspot — has nearly all the participants enrolled in its COVID-19 vaccine trial.
The UI is one of about 120 sites worldwide selected to test a vaccine manufactured by drug companies Pfizer and BioNtech.
The UI began enrolling volunteers for the trial in July, aiming to recruit 250 participants. Now, the trial has about 220 volunteers, 120 of whom have already received a second dose of the vaccine, and the UI plans to recruit its final volunteers by Sept. 23. That date was extended after Iowa City saw a sharp spike in COVID-19 cases as thousands of students returned to campus in August, and made The New York Times list for top outbreaks among metro cities.
Between 20,000 and 30,000 people nationwide will be enrolled in the trial of the vaccine, making the UI just a small segment of the trials. Pfizer announced Sept. 5 the company had enrolled more than 25,000 participants in its trials.
The vaccine is administered by injection. Half of the people in the trial received a salt water placebo and half received the vaccine. Two important components when studying the vaccine, lead University of Iowa researcher Patricia Winokur said, are the efficacy — or how effective the vaccine is in preventing the COVID-19 virus — and the vaccine’s safety.
And being in a location like Iowa City helps vaccine researchers assess better whether the vaccine is effective.
“Being in a hot spot is advantageous because our people participating in the vaccine study are going to be coming in contact more readily with COVID-19 and perhaps showing that the vaccine is superior to what we call a placebo,” Winokur said.
Because the UI study is a fraction of the larger study, the UI group won’t be able to draw conclusions from it. But, Winokur said so far two people in the study tested positive for the virus — though because the study was conducted blindly, they didn’t know whether those people received the vaccine or the placebo.
She added that people in the trial reported few side effects — just having a sore arm, and feeling a little rundown.
The timeline of a vaccine is still uncertain, said Steven Button, emergency preparedness coordinator for Johnson County Public Health, said.
In initial direction from the Iowa Department of Public Health, it’s preparing to distribute a vaccine as soon as November, Button said, though that doesn’t necessarily mean one will be available to everyone at that time.
A vaccine, once one is available, will be distributed in waves, Button said. Initial guidance directs vaccines to be distributed first to health-care workers, those at highest risk of the disease, and those returning to work where social distancing isn’t possible or risk of spread is high, such as meat packing plants, nursing homes, and long-term care facilities.
Button said the department was waiting on exact instructions from the Iowa Department of Public Health and Centers for Disease Control and Prevention.
Speculation about when a vaccine may arrive is swirling in the media, with President Trump asking for a vaccine by Election Day, and others saying one may not be widely available until 2021.
Winokur, too, said it’s impossible to know for sure when a vaccine may arrive. The FDA first has to approve a vaccine before it can be distributed in a phased process.
“The front runners might have approval in December. And then it’s going to be a matter of having a vaccine and having it distributed around the country,” Winokur said. “And that’s going to take many months.”
Several drug manufacturers, such as Pfizer, are taking steps to speed along the process, called Operation Warp Speed. Its goal, according to the Department of Health and Human Services, is to deliver 300 million doses of a safe, effective vaccine for COVID-19 by January 2021.
But, Winokur said, that doesn’t mean the trials are skipping any steps in the approval process. Several rivaling drug manufacturers pledged to dedicate the appropriate time and resources to creating a vaccine in order to build public trust for trials of the vaccine.
Winokur said that companies, such as Pfizer, are starting to manufacture the vaccines upfront before they have data back on the efficacy of vaccines, so if the FDA approves the vaccine, companies are ready to begin distribution as soon as possible.
“If their vaccines fail, and FDA does not approve it, they will throw those vials in the garbage can,” Winokur said.
Organizations, governments, and others are holding out hope that life will return to normal once a vaccine is developed.
The UI announced its plan to continue hybrid instruction during the spring semester, and eliminate spring break.
“While we are confident there will be a COVID-19 vaccine in the near future, it will take time to administer widely,” said Campus Health Officer Dan Fick. “Therefore, we believe it will still be necessary to take steps to mitigate transmission of the virus including maintaining a blended model of instruction and eliminating spring break.”