UI researchers’ findings on cell movement may inform new cancer treatments
University of Iowa researchers in the department of anatomy and cell biology’s Tootle Lab discovered the protein fascin regulates cell stiffness, which promotes cell movement. The finding could have implications for preventing cancer in the future.
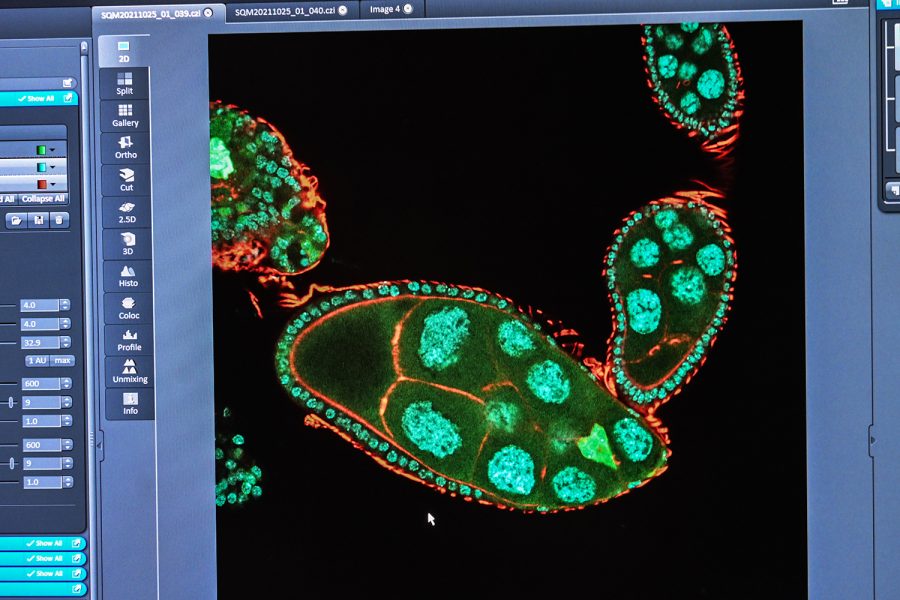
A fly’s slivered ovary is seen on the screen of a confocal lens on November 7, 2021. The green triangle-shaped object on the bottom right is being studied.
November 9, 2021
A group of University of Iowa researchers at the Carver College of Medicine found a cell function that could inform future cancer and birth defect treatment.
The study, published in the journal eLife on Oct. 26, suggests that the protein fascin is responsible for regulating the protein myosin, which stiffens other cells, contributing to the development of cancer when these stiff cells surround cancerous cells.
Tina Tootle, associate professor of anatomy and cell biology at the college of medicine and co-author on the paper, said her team expected to discover that other cells composing on the surface of where the migratory cells — which move through the body and promote the spread of cancer — are responsible for regulating their own stiffness.
“What we found is that fascin is acting in the migrating cells,” she said. “That controls the stiffness or hardness of the surrounding cells and promotes the migration.”
Tootle said cancer cells have been known to move across hard surfaces and suggested that manipulation of cell-stiffening proteins might be a way to inhibit the movement of cancer cells.
By doing so, she said, scientists might artificially stiffen the whole three-dimensional space through which cancer cells move, as opposed to a selective pathway that promotes proper movement, which fascin naturally stiffens.
“I knew going in that the stiffness of the migrating cells and the surrounding cells played a role in cancer and cell migration,” Lamb said.
Fascin carries a tight bundle of actin filaments, she said, which myosin pulls on to control the hardness of surrounding cells, creating a surface suitable for movement.
Tootle said researchers discovered this by deactivating the fascin protein, which led to increased stiffness in both the migratory cell and the surrounding cells.
“It’s easier to move on a sidewalk or a road than it is on wet sand or mud, and so the same thing has been thought for cells as they’re moving,” she said.
Maureen Lamb, the lead author on the paper and a former UI graduate student in Tootle’s lab, said actin filaments are like the scaffolding of the cell.
“It gives the cell its shape and its structure,” she said. “It changes and rearranges and reorganizes a lot to enable a cell to move, and what fascin does is enable those actin pieces to be connected to one another to form larger fibers.”
In addition to cancer treatment, Tootle said her research might be relevant to understanding the causes of birth defects, such as those in the digestive system or cleft lip and cleft palate.
Alexei Tivanski, UI chemistry associate professor and researcher on the project, provided an essential piece to the puzzle: a technique called atomic force microscopy.
“We provided specific values for what is called stiffness, so it tells you how stiff, or hard, or soft a particular cell is,” he said.
Atomic force microscopy, he said, is performed using a sharp tip that is pushed into a cell. The pushback of which is used to determine the hardness of the cell’s surface. The more pushback, the stiffer the cell, he explained.
One of the novel features of this research, Tootle said, was using an organic surface, the ovaries of fruit flies, which is an improvement over using an artificial surface.
“This is the first time that anyone’s looked at whether fascin is regulating myosin and sort of cellular stiffness in a native context, so in a normal environment,” Tootle said. “So much of what we know has come from cells moving as single cells on artificial surfaces like glass and plastic. While it’s told us a lot, it hasn’t told us how cells really move in the body.”