University of Iowa advancing in Novavax COVID-19 vaccine trial
: The University of Iowa Hospitals and Clinics is participating in another COVID-19 vaccine clinical trial, this time with Novavax. This phase three study is another effort made by the hospital to prevent the spread of virus on a global scale.
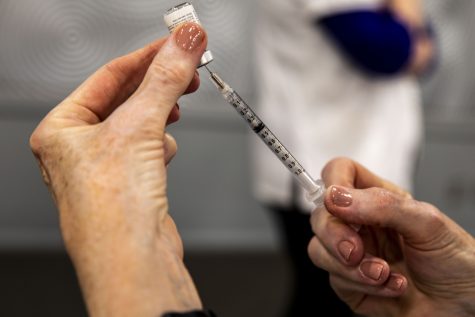
A member of UIHC staff fills needles with the Moderna vaccine on Friday, Jan. 29, 2021 at the UI Medical Education Research Facility.
March 2, 2021
The University of Iowa Hospitals and Clinics is participating in another clinical trial to combat the spread of COVID-19 using vaccines, this time with Novavax, Inc.
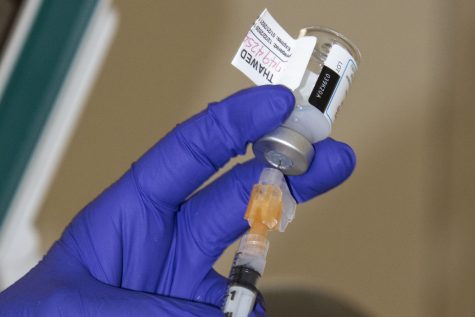
The Phase 3 Trial of a COVID-19 vaccine created by Novavax, Inc., of Gaithersburg, Maryland started in December. According to the National Institute of Health, the trial is planning to enroll 30,000 participants at 115 sites in the U.S. and Mexico.
As previously reported by The Daily Iowan, UIHC served as a trial site for the Pfizer-BioNTech vaccine last year and recruited 250 participants for the double-blind study that vaccinated half of the participants.
Executive Dean of the Carver College of Medicine Pat Winokur, who is UIHC’s vaccine lead researcher, said the Novavax trial studies a more traditional vaccine.
“It’s nice because I think there are some people that are worried about messenger RNA vaccines, and now we have an opportunity to study a more traditional vaccine that people might be comfortable with if they’re worried about the messenger RNA technology,” Winokur said.
The Novavax vaccine is a subunit vaccine, which means it delivers the ready-made spike protein in the form of a virus-like nanoparticle. Pfizer-BioNtech and Moderna vaccines are both messenger RNA vaccines, that use cells to make the spike protein so the immune system can react and make antibodies to the spike protein. These antibodies protect people against the virus if they’ve been exposed after vaccination.
None of the vaccines use a whole or weakened coronavirus, so none of them can cause COVID-19.
Additionally, the Novavax vaccine can be stored at refrigerated temperatures, instead of the subzero temperatures that Moderna and Pfizer-BioNtech require for their vaccines, Winokur said.
“This is a much more practical vaccine for getting out into private practices, rural areas, or other countries that don’t have the infrastructure that the U.S. might have,” she said.
Studying new vaccines is important for many reasons, including being able to distribute a COVID-19 vaccine to more people, Winokur said. She said it can also help people who have severe allergic reactions to different types of vaccines.
“As we study these vaccines, we learn that perhaps some people have allergies that are more likely to occur with one vaccine or another,” Winokur said. “Having more vaccines helps us have a selection of vaccines that we might be able to select for people that have specialized circumstances.”
The phase three study uses a randomized algorithm and has a 2-to-1 ratio for people who get the vaccine – two people get the vaccine for every person who gets the placebo, Winokur said. Researchers will follow trial participants for two years after they receive both doses of the vaccine, she said.
Winokur said researchers will look at the efficacy and safety of the vaccine, as well as whether it can prevent someone from getting COVID-19. If a patient has COVID-19 signs or symptoms, she said researchers will bring them in to test which elements of the virus that they have.
Researchers are studying individuals who are over 18 years old. Winokur said they are prioritizing participants who are 65 and older, and there have been over 1,000 people who have expressed interest.
The Novavax vaccine will need to have a 50 percent efficacy rate before the FDA reviews it for emergency use authorization, Winokur said. She said she expects the vaccine won’t be ready for the public until at least April.
Trial participant Dawn Goodlove from Marion, Iowa said she joined after reading an article about the trial.
She said she joined the trial in December because she felt helpless in the face of the pandemic.
“I truly believe that vaccines are the way out of this pandemic,” Goodlove said. “The idea of being able to contribute to a solution to this huge, global problem was a huge motivator for me.”
Winokur said Goodlove was one of the 55 people enrolled in the trial at the end of January.
Goodlove said she’s enjoyed participating in the trial because of how well the UIHC team has treated her. She said she is hopeful that the Novavax trail will contribute to ending the pandemic.
Goodlove said she recommends everyone participate however they can to stop the spread of COVID-19.
“We’re talking about a major research institution that has a strong track record for contributing to global health and solutions to health issues,” Goodlove said. “Working with the University of Iowa, I have no qualms about it and I don’t think anyone else should either.”
Editor’s note: This article has been updated to note that the Novavax vaccine is a subunit vaccine, meaning it delivers a ready-made spike protein in the form of a virus-like nanoparticle. It does not use an adenovirus to deliver genetic instructions to create the coronavirus spike protein.