Iowa Medical Innovations Group members sell devices to multinational companies
Former members of an Iowa Medical Innovations Group licensed three medical technologies to multinational companies. The technologies, conceptualized at the University of Iowa, consist of a catheter, oximeter, and IV placement system.
January 27, 2022
Inventors formerly involved in the Iowa Medical Innovations Group at the University of Iowa licensed three medical technologies to multinational companies through the LLC, BloodWorks.
The three companies that bought these devices are Teleflex, Getinge, and Becton Dickinson and Company. The Iowa Medical Innovation Group collates members from the colleges of Law, Business, Medicine, and Engineering to conceptualize and invent new and in-need medical devices.
Two of the three technologies alleviate the difficulties of inserting and maintaining catheters for both patients and nurses, explained Han Jun Kim, former UI undergraduate in Biomedical Engineering, former Iowa Medical Innovations Group member, and co-founder of BloodWorks.
Robert Anderson, former health care professional mentor of the Iowa Medical Innovations Group project and co-founder of BloodWorks, said the team’s goal was to translate the deficiencies of medical technology existing or not into functional technologies for medical practitioners.
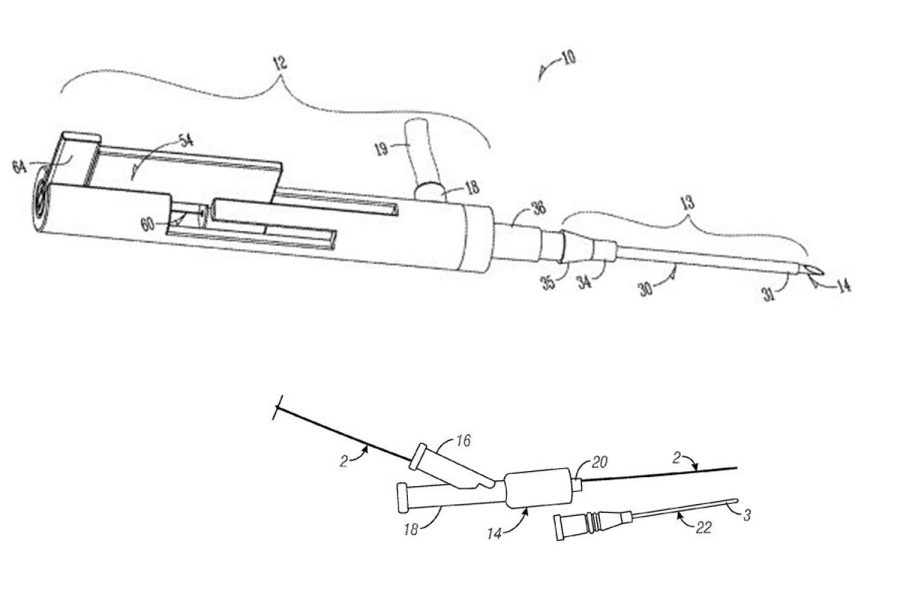
Their first technology, the Segmented Stabilization System, a vein catheter that unfolds in three incremental sizes, began in 2012 at the UI’s Iowa Medical Innovations Group.
Incremental insertion, Kim explained, prevents the needle from puncturing small or weak veins and ensures it slips through properly.
“It’s intended for highly dehydrated patients who have relatively weaker vein walls compared to a healthy patient and also patients who have very small veins,” he said.
“The first step is pretty stiff, the second step is less stiff, and the third step is more flexible,” he said.
Cody Connor, former UI medical graduate student, Iowa Medical Innovations Group member, and BloodWorks co-founder, did not join the group until the team began work on its second project, the Intravascular Oximeter, a device he described as a replacement for faulty fingertip pulse oximeters.
Connor said the current pulse oximeters that clamp to fingertips read oxygen saturation through the skin.
“Sometimes that is not going to be accurate,” he said, “because sometimes patients are very sick and the blood flow isn’t going to extremities like the fingers and the toes. All the time those things are falling off and they result in alarms.”
The new oximeter is meant to be situated in the blood in an inserted catheter. It directly, not indirectly, reads the oxygen saturation, he said.
The final medical device was not created until the group branched from Iowa Medical Innovations Group to the LLC BloodWorks in 2014, Anderson said. It is an IV placement verification system, called IVPlacedIt.
IVPlacedIt tracks whether a catheter or IV is still inserted in the correct veins, Connor said. He explained how catheter slippage can result in extravasation, the leakage of fluids into the tissue surrounding the vessel or vein. Their product spots the placement of the catheter and so prevents such leaks from happening.
Connor said the device can also help nurses build confidence during IV training.
“If nurses are continually getting positive readings from the device saying they got good access [to the vein], that’s going to build their confidence as they are becoming nurses,” he said.
Kim said his involvement in BloodWorks ended about two months ago because he now works as a software engineer at a medical device company.
“We all had our own daytime jobs, and we were all passionate people who wanted to see how our technologies could improve patient care,” he said. “So with that enthusiasm, we just continued to pursue the BloodWorks activity on the side.”
Kim explained each Iowa Medical Innovations Group team has one advisor, three engineering students, two law students, two business students, and one health care worker who is either a doctor or else somehow affiliated with UI Hospitals and Clinics.
“In a medical device startup, since you don’t have many engineers on the team, one person has to put on a lot of different hats, in terms of part of the product development life cycle,” Kim said.
This multiplicity of work gave him a better appreciation for different disciplines, he said. “A lot of the good ideas actually came from law students who had a very different approach,” Kim said.
Kim said participating in Iowa Medical Innovations Group helped students learn to apply their class knowledge to the real world. The program ended two years ago.
Anderson similarly praised Iowa Medical Innovations Group for teaching his team how to bring a medical device from “ideation to commercialization,” and to consider the business, legal, medicine, and engineering aspects of a single product.
“A group of people with varied skillsets and experience and with very limited resources and know-how were able to conceptualize three technologies and take all three of those all the way and transfer to the medical device industry,” he said. “Anybody who puts the work into it and believes in what they’re doing can make a difference in the world.”
Editors Note: In a previous edition of this story, The Daily Iowan did not include the Iowa Medical Innovations Group program coming to an end. The DI regrets this error.